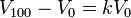1. Acid-a compound with a taste that is sour, makes blue litmus paper red, and is a H+ ion.
2. Neutralization-a reaction that happens between a base and an acid that yields a salt and water
3. Indicator-a thing, like litmus, that tells the presence of a certain constituent like an acid or a base.
4. Corrosive-eating away at something (acids are this)
5. Hydroxide ion-an anion OH that has one oxegen and one hydrogen atom
Cites:
dictionary.com
Thursday, December 2, 2010
Sunday, November 28, 2010
Objective 5:
1. They are mechanical and chemical.
http://wiki.answers.com/Q/What_are_the_two_parts_of_digestion&isLookUp=1
2. Mechanical digestion is when you hve to do something to digest your food for yourself like chewing but chemical digestion is when a chemical called hydrochloric acid breaks down your food. http://wiki.answers.com/Q/What_is_the_difference_between_mechanical_digestion_and_chemical_digestion#ixzz16btmgJUm
3. This means they can't do mechanical digestions so the chemical digestion has to work harder.
4. mounth=7pH, stomach acid=1.5pH, small intestine=7
http://wiki.answers.com/Q/What_is_the_ph_in_your_mouth&isLookUp=1
http://wiki.answers.com/Q/What_is_the_pH_of_the_stomach
http://wiki.answers.com/Q/What_is_the_Ph_of_the_small_intestine&isLookUp=1
5. The different enzymes work better at different pHs.
http://wiki.answers.com/Q/How_is_pH_important_during_digestion&alreadyAsked=1&rtitle=Why_are_pH_variations_in_different_parts_of_the_digestive_system_important_to_the_process_of_digestion&isLookUp=1
http://wiki.answers.com/Q/What_are_the_two_parts_of_digestion&isLookUp=1
2. Mechanical digestion is when you hve to do something to digest your food for yourself like chewing but chemical digestion is when a chemical called hydrochloric acid breaks down your food. http://wiki.answers.com/Q/What_is_the_difference_between_mechanical_digestion_and_chemical_digestion#ixzz16btmgJUm
3. This means they can't do mechanical digestions so the chemical digestion has to work harder.
4. mounth=7pH, stomach acid=1.5pH, small intestine=7
http://wiki.answers.com/Q/What_is_the_ph_in_your_mouth&isLookUp=1
http://wiki.answers.com/Q/What_is_the_pH_of_the_stomach
http://wiki.answers.com/Q/What_is_the_Ph_of_the_small_intestine&isLookUp=1
5. The different enzymes work better at different pHs.
http://wiki.answers.com/Q/How_is_pH_important_during_digestion&alreadyAsked=1&rtitle=Why_are_pH_variations_in_different_parts_of_the_digestive_system_important_to_the_process_of_digestion&isLookUp=1
Objective 4:
1. H+
http://wiki.answers.com/Q/What_ion_is_present_in_all_acidic_solutions
2. The acids make hydrogen ions and the bases make hydroxide ions.
http://wiki.answers.com/Q/What_happens_when_acids_and_bases_dissolve_in_water
3. It ends up breaking down into H+ NO3 which is an acid.
http://answers.yahoo.com/question/index?qid=20080108190009AA0Uied
4. The pH tells you how much H+ ions that are inside of a certain acid or base. If the pH is lower than 7 it is an acid and if it is higher it is a base.
(remembered this from the question on objective 3)
5. The concentration of hydrogen is pretty high when the pH is low which means the pH of 3 has more hydrogen ions than the pH of 7.
http://wiki.answers.com/Q/What_solution_has_a_greater_concentration_of_hydrogen_ions_a_solution_with_a_pH_of_3_of_a_pH_of_7_Explain
http://wiki.answers.com/Q/What_ion_is_present_in_all_acidic_solutions
2. The acids make hydrogen ions and the bases make hydroxide ions.
http://wiki.answers.com/Q/What_happens_when_acids_and_bases_dissolve_in_water
3. It ends up breaking down into H+ NO3 which is an acid.
http://answers.yahoo.com/question/index?qid=20080108190009AA0Uied
4. The pH tells you how much H+ ions that are inside of a certain acid or base. If the pH is lower than 7 it is an acid and if it is higher it is a base.
(remembered this from the question on objective 3)
5. The concentration of hydrogen is pretty high when the pH is low which means the pH of 3 has more hydrogen ions than the pH of 7.
http://wiki.answers.com/Q/What_solution_has_a_greater_concentration_of_hydrogen_ions_a_solution_with_a_pH_of_3_of_a_pH_of_7_Explain
Objective 3:
1. They are if something tastes sour, if it reacts to metals or carbonates, if it turns blue litmus paper red, a pH lower than 7, and lastly if in a solution it produces hydrogen ions.
http://wiki.answers.com/Q/What_properties_distinguish_acids&alreadyAsked=1&rtitle=What_are_four_properties_of_acids&isLookUp=1
2. They taste bitter, feel slippery, can be caustic, and turn red litmus paper blue.
http://wiki.answers.com/Q/What_are_the_properties_of_bases#ixzz16botVnjO
3. If the paper turns red it must be an acid and if it turns it blue it must be a base.
4. You can tell because if the food is sour it is a acid and if it is bitter it is a base.
5. You need to wear gloves because the fertilizer will hurt your hands. Also allows you not to wash your hans so often while doing gardening.
http://wiki.answers.com/Q/Why_is_it_wise_to_wear_gloves_while_spreading_fertilizer_in_a_garden
http://wiki.answers.com/Q/What_properties_distinguish_acids&alreadyAsked=1&rtitle=What_are_four_properties_of_acids&isLookUp=1
2. They taste bitter, feel slippery, can be caustic, and turn red litmus paper blue.
http://wiki.answers.com/Q/What_are_the_properties_of_bases#ixzz16botVnjO
3. If the paper turns red it must be an acid and if it turns it blue it must be a base.
4. You can tell because if the food is sour it is a acid and if it is bitter it is a base.
5. You need to wear gloves because the fertilizer will hurt your hands. Also allows you not to wash your hans so often while doing gardening.
http://wiki.answers.com/Q/Why_is_it_wise_to_wear_gloves_while_spreading_fertilizer_in_a_garden
Objective 2:
1. Concentration is the measurment of what amount of one thing is in another thing. To figure this out you need to know how the solvent is compared to the solute.
http://en.wikipedia.org/wiki/Concentration
2. Solubility is used to idenify substances because it is a characteristic property of matter.
http://wiki.answers.com/Q/Why_is_solubility_useful_in_identifying_substances#ixzz16bmbSKQR
3. There are three types and those are tempature, pressure, and type of the solvent.
http://wiki.answers.com/Q/What_factors_affect_the_solubility_of_a_substance&isLookUp=1
4. When the tempature is hot the solid will melt and if it is too cold it will freeze.
http://wiki.answers.com/Q/How_does_temperature_affect_the_solubility_of_a_solid
5. It can help because of the object's characteristic property of matter. This helps you know how easily something may be able to dissolve inside of that certain matter.
http://wiki.answers.com/Q/How_can_solubility_help_you_identify_a_substance&isLookUp=1
http://en.wikipedia.org/wiki/Concentration
2. Solubility is used to idenify substances because it is a characteristic property of matter.
http://wiki.answers.com/Q/Why_is_solubility_useful_in_identifying_substances#ixzz16bmbSKQR
3. There are three types and those are tempature, pressure, and type of the solvent.
http://wiki.answers.com/Q/What_factors_affect_the_solubility_of_a_substance&isLookUp=1
4. When the tempature is hot the solid will melt and if it is too cold it will freeze.
http://wiki.answers.com/Q/How_does_temperature_affect_the_solubility_of_a_solid
5. It can help because of the object's characteristic property of matter. This helps you know how easily something may be able to dissolve inside of that certain matter.
http://wiki.answers.com/Q/How_can_solubility_help_you_identify_a_substance&isLookUp=1
HW 10:
Objective 1: Understanding Solutions
1. What are the characteristics of solutions, colloids, and suspensions? Suspensions are a "homogeneous fluid" that has solid particals that are large. Colloids disperse in a substance evenly and solutions have much smaller particals than either of the other two.
http://en.wikipedia.org/wiki/Solutions
http://en.wikipedia.org/wiki/Colloids
http://en.wikipedia.org/wiki/suspensions
2. What happens to the particles of a solute when a solution forms? The particals from the solute go away and are a part of the solevent.
http://zellescienceblog.blogspot.com/
3. How do solutes affect the freezing point and boiling point of a solvent? The boiling point of the solvent gets bigger when a solute is added because a solution has a bigger boiling point compared to a pure solvent. The freezing point gets lower also.
http://eigthscience.blogspot.com/
http://eigthscience.blogspot.com/
5. What effects do solutes have on a solvent’s freezing and boiling points? A solute makes the boiling point bigger because a solution has a bigger boiling point compared to a pure solvent. It also makes the freezing point a lot lower. The coligative property helps to understand the boiling point elevation.
http://en.wikipedia.org/wiki/Boiling-point_elevation4. Suppose you mix food coloring in water to make it blue. Have you made a solution or solution or a suspension? Explain. You would make a solution because it is going throughout the water and dissolves.
1. What are the characteristics of solutions, colloids, and suspensions? Suspensions are a "homogeneous fluid" that has solid particals that are large. Colloids disperse in a substance evenly and solutions have much smaller particals than either of the other two.
http://en.wikipedia.org/wiki/Solutions
http://en.wikipedia.org/wiki/Colloids
http://en.wikipedia.org/wiki/suspensions
2. What happens to the particles of a solute when a solution forms? The particals from the solute go away and are a part of the solevent.
http://zellescienceblog.blogspot.com/
3. How do solutes affect the freezing point and boiling point of a solvent? The boiling point of the solvent gets bigger when a solute is added because a solution has a bigger boiling point compared to a pure solvent. The freezing point gets lower also.
http://eigthscience.blogspot.com/
http://eigthscience.blogspot.com/
5. What effects do solutes have on a solvent’s freezing and boiling points? A solute makes the boiling point bigger because a solution has a bigger boiling point compared to a pure solvent. It also makes the freezing point a lot lower. The coligative property helps to understand the boiling point elevation.
http://en.wikipedia.org/wiki/Boiling-point_elevation4. Suppose you mix food coloring in water to make it blue. Have you made a solution or solution or a suspension? Explain. You would make a solution because it is going throughout the water and dissolves.
Monday, November 22, 2010
Science Test by Ansley and Nora
F C K Person Object
95 35 308.15 Jiro Wang We are 95 degrees
77 25 298.15 Calvin Chen At this store and it was 77 degrees
59 15 288.15 Wu Chun My house was 59 on last Thursday night
41 5 278.15 Aaron Yan Changed water to 41 degrees in sink
Friday, November 19, 2010
Test 9:
- Thermal energy is the total energy of all of the particles in one object while temperature and heat is an thermal energy moving from a hotter object to a colder one like when I touch a rock that has been in the snow for two days I am passing heat to it. Temperature is the measure of the warmness or the coldness of some object compared to a set value.
- Some things get hotter faster than others because their specific heat is a lot more or a lot less than another object.
- One type of heat transfer is conduction and this is when heat transfer is from one place to another without the movement of any matter. These transfer thermal energy very well and are many types of silver and other things like that. The reason why silver and those things are conductors are because they are cold to the touch. They are this way because it is the way the particles are arranged. Another type is convection and this is when movement of heat transfer is by movement. These things don’t transfer thermal energy well at all which makes them good for keeping you warm. That is why they aren’t cold to the touch like conductors. Some examples are clothes, blankets, and wool socks because these things don’t transfer the thermal energy well. The last type is radiation and that is the transfer of thermal energy by electromagnetic waves. Some examples are radio waves, microwave waves, and other object like this. As humans we can’t see these, we just see a small part of this which is the visible light section.
- You need to raise the temperature by 209,000J.
- A tent would trap the air you are in and make it warmer which would be conduction. The fire would send out heat to make you warm so that would be radiation. Both of these would help but a fire would help the most because you would get warmer right away while being in your tent wouldn’t help right away, it would take a long time. That is why I would build a fire and not set up a tent.
Sites I Used
http://ansleydevore.blogspot.com/
http://griffinscience.com/
http://dictionary.reference.com/browse/temperature
Wednesday, November 17, 2010
Objective 5:
1. They use the thermal energy by making the gas getting bigger inside of this cylender shaped thing. This makes this thing called the pistol to go downward causing there to be a spark which then lights the spark plug.
http://www.ehow.com/how-does_4926292_car-engine-piston-work.html
http://wiki.answers.com/Q/In_a_heat_engine_what_happens_to_thermal_energy_that_is_converted_from_chemical_energy
2. They are alike and different becuase of what it this says "In an internal combustion engine, a working fluid is heated and expanded to produce power, captured in a cylinder and piston or in a turbine or similar system, and this heating and expansion is produced by burning fuel inside the expansion chamber. In an external combustion engine, the working fluid is heated by burning fuel, then introduced into the expansion chamber to produce power." from http://wiki.answers.com/Q/Difference_between_internal_combustion_engines_and_external_combustion_engines. This basically means that the external one makes power by fluid being heated up while the internal one is made by a fluid that is heated and expands to make power.
http://wiki.answers.com/Q/Difference_between_Internal_combustion_and_external_combustion_engine
3. I think that most likely (even though I now nothing about cars) that the external one could be a little bit dangerous becuase it is done by burning fuel which could be dangerous.
4. What happens it all the hot air is sucked out causing it to be very cold inside of it.
5. It would effect it because then all of the hot air would stay in there and cause everything to be getting hotter.
http://www.ehow.com/how-does_4926292_car-engine-piston-work.html
http://wiki.answers.com/Q/In_a_heat_engine_what_happens_to_thermal_energy_that_is_converted_from_chemical_energy
2. They are alike and different becuase of what it this says "In an internal combustion engine, a working fluid is heated and expanded to produce power, captured in a cylinder and piston or in a turbine or similar system, and this heating and expansion is produced by burning fuel inside the expansion chamber. In an external combustion engine, the working fluid is heated by burning fuel, then introduced into the expansion chamber to produce power." from http://wiki.answers.com/Q/Difference_between_internal_combustion_engines_and_external_combustion_engines. This basically means that the external one makes power by fluid being heated up while the internal one is made by a fluid that is heated and expands to make power.
http://wiki.answers.com/Q/Difference_between_Internal_combustion_and_external_combustion_engine
3. I think that most likely (even though I now nothing about cars) that the external one could be a little bit dangerous becuase it is done by burning fuel which could be dangerous.
4. What happens it all the hot air is sucked out causing it to be very cold inside of it.
5. It would effect it because then all of the hot air would stay in there and cause everything to be getting hotter.
Sunday, November 14, 2010
Objective 4:
1. Something changes state when it gets hotter or is heated up.
http://wiki.answers.com/Q/What_causes_matter_to_change_state
2. It causes the paritcles to move faster and after awhile it will become a liquid if it is a solid.
http://wiki.answers.com/Q/What_happens_to_the_particles_of_a_solid_as_the_thermal_energy_of_the_substance_increases
3. The tempature stays the same because all the tempature's energy is being put into the matter changing in its state. http://wiki.answers.com/Q/Why_does_the_temperature_of_matter_remain_the_same_while_the_matter_changes_state
4. When something gets hotter it begins to melt becuase all of the matter gets bigger causing making it melt away.
http://wiki.answers.com/Q/What_causes_a_solid_to_melt
5. It is mainly made from moisture so as it is heated up it begins to steam. If the steam doesn't have a way to get out then it will explode.
http://wiki.answers.com/Q/Why_should_you_poke_holes_in_a_potato_before_baking_it
http://wiki.answers.com/Q/What_causes_matter_to_change_state
2. It causes the paritcles to move faster and after awhile it will become a liquid if it is a solid.
http://wiki.answers.com/Q/What_happens_to_the_particles_of_a_solid_as_the_thermal_energy_of_the_substance_increases
3. The tempature stays the same because all the tempature's energy is being put into the matter changing in its state. http://wiki.answers.com/Q/Why_does_the_temperature_of_matter_remain_the_same_while_the_matter_changes_state
4. When something gets hotter it begins to melt becuase all of the matter gets bigger causing making it melt away.
http://wiki.answers.com/Q/What_causes_a_solid_to_melt
5. It is mainly made from moisture so as it is heated up it begins to steam. If the steam doesn't have a way to get out then it will explode.
http://wiki.answers.com/Q/Why_should_you_poke_holes_in_a_potato_before_baking_it
Objective 3:
1. They are conduction, convection, and radiation.
http://wiki.answers.com/Q/What_are_3_forms_of_heat_transfer&alreadyAsked=1&rtitle=What_are_the_three_forms_of_heat_transfer
2. It always lets go of heat never lets go of coldness.
http://wiki.answers.com/Q/In_which_direction_is_heat_transferred
3. Conductors make it easy for energy to flow through it and insulators cause the energy to go slower through it. Also conductors are cold at the touch while insulators keep things warm such as wool socks.
4. I would say that it would be better to use the copper pipe as an conductor because it is cold at the touch and it allows things to to flow easily.
5. I would first get in my tent (conduction) because trapped air makes warm air but the fire (radiation) helps a lot too becuase it gives off hot air which does help. So I would most likely first get inside my tent till I felt pretty warm and then stuff a lot of leaves and other things into my jacket because that helps keep you warm. Then I would build my fire. The fire would help the most though.
http://www.highsierraadventures.com/skills/survival.htm
http://wiki.answers.com/Q/What_are_3_forms_of_heat_transfer&alreadyAsked=1&rtitle=What_are_the_three_forms_of_heat_transfer
2. It always lets go of heat never lets go of coldness.
http://wiki.answers.com/Q/In_which_direction_is_heat_transferred
3. Conductors make it easy for energy to flow through it and insulators cause the energy to go slower through it. Also conductors are cold at the touch while insulators keep things warm such as wool socks.
4. I would say that it would be better to use the copper pipe as an conductor because it is cold at the touch and it allows things to to flow easily.
5. I would first get in my tent (conduction) because trapped air makes warm air but the fire (radiation) helps a lot too becuase it gives off hot air which does help. So I would most likely first get inside my tent till I felt pretty warm and then stuff a lot of leaves and other things into my jacket because that helps keep you warm. Then I would build my fire. The fire would help the most though.
http://www.highsierraadventures.com/skills/survival.htm
Objective 2:
1. They measure the tempature by telling how fast the paritcles in the air are moving. So if it was a hot tempature that would mean that the particles are moving really fast.
2. Well the Kelvin and Celsius ones both measure in the same degrees between the freezing and boiling points but they have different zero points which make them very alike and different. One intervel of Fahrenheit is equal to about 5/9 of Celsius which shows how they are different. "Unlike the degree Fahrenheit and degree Celsius, the kelvin is not referred to or typeset as a degree." This was said by http://en.wikipedia.org/wiki/Kelvin. And this basically means that Kelvin isn't really a degree such as Celsuis and Fahrenheit.
http://wiki.answers.com/Q/How_are_the_three_temperature_scales_alike
http://en.wikipedia.org/wiki/Fahrenheit
3. 41 F
4. 460 C
5. The tempature needs to be raised by 209,000K
2. Well the Kelvin and Celsius ones both measure in the same degrees between the freezing and boiling points but they have different zero points which make them very alike and different. One intervel of Fahrenheit is equal to about 5/9 of Celsius which shows how they are different. "Unlike the degree Fahrenheit and degree Celsius, the kelvin is not referred to or typeset as a degree." This was said by http://en.wikipedia.org/wiki/Kelvin. And this basically means that Kelvin isn't really a degree such as Celsuis and Fahrenheit.
http://wiki.answers.com/Q/How_are_the_three_temperature_scales_alike
http://en.wikipedia.org/wiki/Fahrenheit
3. 41 F
4. 460 C
5. The tempature needs to be raised by 209,000K
Objective 1:
1. The three types are Fahrenheit, Celsius, and Kelvin scales.
http://wiki.answers.com/Q/What_are_the_names_of_three_temperature_scales
2. The thermal energy is what causes the temperature to change because when the energy isn't very strong than it is cold outside but when it is strong it is hot outside because the molecule get heated up and energized from the thermal energy. The thermal energy is the energy of all of the particles in an object and you need to know the temp. of them and the heat to find this.
3. This basically means the amount of energy that is needed to make a substance get bigger by one degree Celcius. Such as water's specific heat is a lot higher than ice.
http://wiki.answers.com/Q/Why_does_water_have_a_high_and_specific_heat_capacity
4. It will melt in your hand because your hand is very warm compared to the ice which is frozen so since your hand is so warm you melt the ice. The ice doesn't make you colder it is just taking away the heat.
5. There are two things that have to do with why some things get hotter than others faster. One thing is just how much water (mositer) in the object. The other thing is some thinkings have higher or lower specific heat thing that I explained in problem 3. If it has a higher specific heat than it takes a lot longer than if it happened to have a lower one.
http://zellescienceblog.blogspot.com/
http://wiki.answers.com/Q/What_are_the_names_of_three_temperature_scales
2. The thermal energy is what causes the temperature to change because when the energy isn't very strong than it is cold outside but when it is strong it is hot outside because the molecule get heated up and energized from the thermal energy. The thermal energy is the energy of all of the particles in an object and you need to know the temp. of them and the heat to find this.
3. This basically means the amount of energy that is needed to make a substance get bigger by one degree Celcius. Such as water's specific heat is a lot higher than ice.
http://wiki.answers.com/Q/Why_does_water_have_a_high_and_specific_heat_capacity
4. It will melt in your hand because your hand is very warm compared to the ice which is frozen so since your hand is so warm you melt the ice. The ice doesn't make you colder it is just taking away the heat.
5. There are two things that have to do with why some things get hotter than others faster. One thing is just how much water (mositer) in the object. The other thing is some thinkings have higher or lower specific heat thing that I explained in problem 3. If it has a higher specific heat than it takes a lot longer than if it happened to have a lower one.
http://zellescienceblog.blogspot.com/
Sunday, November 7, 2010
Objective 6:
1. This law shows how and why gases get bigger when they get hotter.
http://en.wikipedia.org/wiki/Charles%27_Law
2. As the temperature of a gas increases, the gas molecules move more crazily because they get more thermal energy.
3. The Robert brothers were the first to build one of these for Charels and then Charels flew in it first. http://en.wikipedia.org/wiki/Robert_brothers
4. When it landed local people attacked it. They wree afraid of it and so ripped it up with knives and other things.
http://en.wikipedia.org/wiki/Robert_brothers
5. The k is kept constant in the formula:
http://en.wikipedia.org/wiki/Charles_law
http://en.wikipedia.org/wiki/Charles%27_Law
2. As the temperature of a gas increases, the gas molecules move more crazily because they get more thermal energy.
3. The Robert brothers were the first to build one of these for Charels and then Charels flew in it first. http://en.wikipedia.org/wiki/Robert_brothers
4. When it landed local people attacked it. They wree afraid of it and so ripped it up with knives and other things.
http://en.wikipedia.org/wiki/Robert_brothers
5. The k is kept constant in the formula:
http://en.wikipedia.org/wiki/Charles_law
Objective 5:
1. This law shows the relationship between pressure and volume of a gas.
http://en.wikipedia.org/wiki/Boyle%27s_Law
2. As the balloon rises it begins to get bigger which means that they can't fill a balloon completely up or else it will pop.
http://wiki.answers.com/Q/Why_do_most_helium_filled_balloons_burst_as_they_ascend_to_high_altitudes
3. PV = k is the formula. P=pressure V=volume of gas k=constant value of pressure and volume
http://wiki.answers.com/Q/What_is_the_formula_of_Boyle's_law&alreadyAsked=1&rtitle=What_is_the_formula_for_Boyle%27s_Law
4. They use it when they do oxegen tanks because when they find the pressure in the tank they can see how much is actually left in the tank.
5. Scuba divers use it for their oxegen tanks because just like doctors they can see how much oxegen they have left.
http://en.wikipedia.org/wiki/Boyle%27s_Law
2. As the balloon rises it begins to get bigger which means that they can't fill a balloon completely up or else it will pop.
http://wiki.answers.com/Q/Why_do_most_helium_filled_balloons_burst_as_they_ascend_to_high_altitudes
3. PV = k is the formula. P=pressure V=volume of gas k=constant value of pressure and volume
http://wiki.answers.com/Q/What_is_the_formula_of_Boyle's_law&alreadyAsked=1&rtitle=What_is_the_formula_for_Boyle%27s_Law
4. They use it when they do oxegen tanks because when they find the pressure in the tank they can see how much is actually left in the tank.
5. Scuba divers use it for their oxegen tanks because just like doctors they can see how much oxegen they have left.
Objective 4:
1. The warmer particles are bouncing around everywhere because of the energy that the sun is giving out. This makes them warmer because they have more energy. The cooler particals don't have as much sun energy causing them to be less bouncy and wild so they get cold.
2. Ice-cream starts to melt on warm summer days because when it is cold for them feezer the paritcals are just sort of sitting there and not moving around as much but then the sun causes the particals to get energized so they start to heat up causing the ice-cream to melt.
3. The melting point of particals has a lot to do with the vibration of the particals. This is because as the particals begin to move around a lot they get a lot hotter causing the object to melt.
4. This happens when it is really humid in the air and then all the sudden it gets cold. This then makes the air dissolve so then the water is made into little drops of water like on a hot day and you see condensation on the cup.
http://wiki.answers.com/Q/Why_does_condensation_occur
5. Sublimation happens when a gas turns to a solid without becoming a liquid. From the website that follows I got this quote thing explaing it. "It is shown that the form of the sublimation process in which phase transformation takes place not from the geometric surface but in a certain layer of finite thickness is a stable one."
http://www.springerlink.com/content/qr74408237k73226/
2. Ice-cream starts to melt on warm summer days because when it is cold for them feezer the paritcals are just sort of sitting there and not moving around as much but then the sun causes the particals to get energized so they start to heat up causing the ice-cream to melt.
3. The melting point of particals has a lot to do with the vibration of the particals. This is because as the particals begin to move around a lot they get a lot hotter causing the object to melt.
4. This happens when it is really humid in the air and then all the sudden it gets cold. This then makes the air dissolve so then the water is made into little drops of water like on a hot day and you see condensation on the cup.
http://wiki.answers.com/Q/Why_does_condensation_occur
5. Sublimation happens when a gas turns to a solid without becoming a liquid. From the website that follows I got this quote thing explaing it. "It is shown that the form of the sublimation process in which phase transformation takes place not from the geometric surface but in a certain layer of finite thickness is a stable one."
http://www.springerlink.com/content/qr74408237k73226/
Thursday, November 4, 2010
Objective 3:
1. There are six types of forms of energy related to they changes in matter. They are kinetic, potential, electromagnetic, chemical, electical, and last but not least thermal energy.
2. Kinetic energy
3. Potential energy
4. Electromagnetic energy is basically things like radiation waves, gamma rays, and other things like that, that we can't see.
5. That energy is electical energy. One example of this is the projector shoots these electrons out to form a picture on the board.
2. Kinetic energy
3. Potential energy
4. Electromagnetic energy is basically things like radiation waves, gamma rays, and other things like that, that we can't see.
5. That energy is electical energy. One example of this is the projector shoots these electrons out to form a picture on the board.
Wednesday, November 3, 2010
Objective 2:
1. Well a chemical and physical change are very different. A chemical change is when something that is inside of something else changes like the particles or mineral things that are in it change. A physical change is something that you can see. It is like if I died my hair green, you can see the difference on the external or the outside of me. http://wiki.answers.com/Q/The_main_difference_between_a_physical_and_a_chemical_change_is&alreadyAsked=1&rtitle=What_is_the_main_difference_between_a_physical_change_and_a_chemical_change
2. A chemical change can occor in four ways. One ways is that a solid is made. Another is that an object changes color. The third way is that gases are formed and made. The last way is that light, sound, and/or heat are made.
http://wiki.answers.com/Q/Four_ways_you_can_tell_a_chemical_change_has_taken_place
3. The law basically said that in a closed object that the mass of that object will stay the same. It was made by ancient Greek philosophy people but one man's name that helped a lot was Epicurus.
http://en.wikipedia.org/wiki/Conservation_of_mass
4. Tempature and thermal energy are very different. Tempature is when how hot something is, is the average of kinetic energy. Themral energy is averaged with kinetic energy and all the other different types of energy.
http://wiki.answers.com/Q/How_is_temperature_and_thermal_energy_different
5. Exothermic-A lizard is exothermic because it uses the sun to get body heat.
Endothermic-We are endothermic because we make our own body heat.
2. A chemical change can occor in four ways. One ways is that a solid is made. Another is that an object changes color. The third way is that gases are formed and made. The last way is that light, sound, and/or heat are made.
http://wiki.answers.com/Q/Four_ways_you_can_tell_a_chemical_change_has_taken_place
3. The law basically said that in a closed object that the mass of that object will stay the same. It was made by ancient Greek philosophy people but one man's name that helped a lot was Epicurus.
http://en.wikipedia.org/wiki/Conservation_of_mass
4. Tempature and thermal energy are very different. Tempature is when how hot something is, is the average of kinetic energy. Themral energy is averaged with kinetic energy and all the other different types of energy.
http://wiki.answers.com/Q/How_is_temperature_and_thermal_energy_different
5. Exothermic-A lizard is exothermic because it uses the sun to get body heat.
Endothermic-We are endothermic because we make our own body heat.
Tuesday, November 2, 2010
Objective One:
Objective 1:
1. Mass is more important for many reasons. One reason, which is the main one, is that if I were to weigh myself on Mars I would have a differet weight than I would on Earth. This shows that the weight of an object can change but the mass is always the same. Just like my mass wouldn't change even if I were on Mars. http://wiki.answers.com/Q/Why_is_mass_more_useful_than_weight_for_measuring_matter
2. the volume is 619.65cm3 (the three is supposed to be very small and on the top but I don't know how to do that)
3. The measurement of density is g/cm3 (same thing up there with the three being small and on the top)
4. D=mass over volume
5. V=L*W*H (* means times)
1. Mass is more important for many reasons. One reason, which is the main one, is that if I were to weigh myself on Mars I would have a differet weight than I would on Earth. This shows that the weight of an object can change but the mass is always the same. Just like my mass wouldn't change even if I were on Mars. http://wiki.answers.com/Q/Why_is_mass_more_useful_than_weight_for_measuring_matter
2. the volume is 619.65cm3 (the three is supposed to be very small and on the top but I don't know how to do that)
3. The measurement of density is g/cm3 (same thing up there with the three being small and on the top)
4. D=mass over volume
5. V=L*W*H (* means times)
Friday, October 22, 2010
Superfluids
Superfluid
The definition of a superfluid is: "a fluid that exhibits frictionless flow, very high heatconductivity, and other unusual physical properties, heliumbelow 2.186 K being the only known example."
Thursday, October 21, 2010
Part 2 of our Endothermic Reaction Project
This is Nora and I doing our experiment on Endothermic Reactions.
http://www.youtube.com/watch?v=iqdtSDNRqnc
http://www.youtube.com/watch?v=iqdtSDNRqnc
Tuesday, October 19, 2010
Endotherm experiment: By Ansley and Nora
This is Nora and I explaining what we will be doing with our experiment. There will be another two post of us doing our experiment and then saying what happened and why that did happen.
http://www.youtube.com/samharrelson#p/a/u/0/OC__yM5CSZg
http://www.youtube.com/samharrelson#p/a/u/0/OC__yM5CSZg
Sunday, October 10, 2010
Last Part of Test 1
It is a little difficult to see in this video but here is me explaining what everything is in our peach pit model by alyssa, elizabeth sexton, and me.
http://www.youtube.com/watch?v=1JdG2n3L3Uk
http://www.youtube.com/watch?v=1JdG2n3L3Uk
Sunday, September 19, 2010
Friday, September 17, 2010
Monday, September 13, 2010
Sunday, September 12, 2010
Atomic Mass
Atomic Mass:
- It is the mass of the protons, electrons, and neutrons in one single atom.
- Unusually it is done by AMUs or atomic mass units.
- Atomic mass is normally said to be the mass of an atom which is the mass of one isotope.
- An isotope is defined by dictionary.com as "any of two or more forms of a chemical element, having the same number of protons in the nucleus, or the same atomic number, but having different numbers of neutrons in the nucleus, or different atomic weights."
- Some people say that atomic mass is similar to relative atomic mass, average atomic mass, and atomic weight but that isn't correct.
- Relative atomic mass is very close to atomic weight and now it is replacing atomic weight
- John Dalton was basically the first person to determine the atomic weight of atoms
Science Test!
https://docs0.google.com/a/sdsgriffin.org/document/edit?id=1x7q1qvdKHwqiJic6j7v7deM5SotOcYERCHVJLO-DOhA&hl=en#
This is the answer to question 1 from test 1!
This is the answer to question 1 from test 1!
Tuesday, September 7, 2010
Sodium Chloride (Extra Credit)
Sodium Chloride is the most interesting compound. The way to write is scientifically is NaCl. It is the most interesting to me for many reasons. One reason I think that they are the most interesting is that it is all around us. One place everyone has seen it is right on their kitchen table. You might not have know this but it is your table salt that you use most likley every day. You might not have know that it is a compound. Another place you can find it is in the ocean. It is the main reason from making the ocean salty. It is also used when their is ice or snow on the road and we just put some Sodium Chloride on it. It also is in a lot of shampoos that we have today. That is why this is so cool is that we use this stuff everyday but really didn't know that it was anything that amazing.
Back to the real facts of Sodium Chloride though. Sodium Chloride is made up of Sodium and Chlorine. It is an ionic compound and that basically just means that the ions are kept together in a networked structure by ionic bonds. NaCl is an oderless and colorless compound that looks like crystals. It is also non-flammable which could be the reason it is the main part in a fire extinguisher that makes it get rid of fire. This is all the reasons why NaCl is the coolest compound out there!!!!!!
(By the way if anyone watches Jimmy Neutron here I would just like to say that in that episode where he is working at this reastraunt and the manager is really stupid, the manager says sweep up that salt and Jimmy is like ok I will get that Sodium Chloride and they get in this fight over what the name of it is because the manager thinks that Jimmy is being like stupid or something!!! Really a great show! Sorry just had to add that in there.)
Back to the real facts of Sodium Chloride though. Sodium Chloride is made up of Sodium and Chlorine. It is an ionic compound and that basically just means that the ions are kept together in a networked structure by ionic bonds. NaCl is an oderless and colorless compound that looks like crystals. It is also non-flammable which could be the reason it is the main part in a fire extinguisher that makes it get rid of fire. This is all the reasons why NaCl is the coolest compound out there!!!!!!
(By the way if anyone watches Jimmy Neutron here I would just like to say that in that episode where he is working at this reastraunt and the manager is really stupid, the manager says sweep up that salt and Jimmy is like ok I will get that Sodium Chloride and they get in this fight over what the name of it is because the manager thinks that Jimmy is being like stupid or something!!! Really a great show! Sorry just had to add that in there.)
Saturday, September 4, 2010
History of the Electrons and Protons
Electrons:
J.J. Tomson was the man that in 1897 discovered that an atom has negatively charged parts. He thought that these negative parts are in the atom just kind of floating around but he was wrong. He also relized that an atom has a neutral charge which means there must be positively charged parts also. But, back to electrons. They have a very small mass compared to the other parts of an atom. It has a mass of 0.000000000000000000000000000911 grams. Is about 1/836 atomic mass units.They are very small and very light and they are nothing compared to the nucleus of an atom. Electrons can be at different energy levels throughout the atom. They can take in or let go of something called a photon. A photon is particles of light. To learn more about these photons go to http://www.windows2universe.org/physical_science/magnetism/photon.html In the atom these electrons don'tgo in little tracks like some people thought. Erwin Shroedinger found out that these electrons just zoom around the atom where ever they want. There isn't a certain circle they follow the whole time. The Modern Atomic Model has a nucleus in the center and then there is a big area where we think the electrons might be. As you can see these electrons are very interesting but here is some information about the proton.
Protons:
Protons are very close to the size of a neutron and a lot bigger than electrons. It has a mass of 1 atomic mass units.It was discovored in 1897 by J.J. Thomson. It has 1,836 times more mass than an electron. They are positively charged and there are the same number as protons as electrons. You can find how many protons by looking at the atomic number in the upper corner of the atom in the periotic table. A proton is made up of these things called quarks. There are three quarks in every proton. They are up, up, and down. There are six types of quarks and they are up, down, top, bottom, charm, and strange. These quarks are held together by gluons. Protons are in the middle of the atom with the neutron. The neutrons and protons make up the nucleus that has all the mass of an atom.
Here is a drawing of an atom. But remeber that the electrons don't go on set circles they go where they want to go and just sort of bonce around.
This shows a proton and the quarks that are inside of it and that are held together by gluons.
Citations:
I got all my information from http://en.wikipedia.org/wiki/Electrons http://en.wikipedia.org/wiki/Proton http://www.windows2universe.org/physical_science/physics/atom_particle/proton.html http://www.windows2universe.org/physical_science/physics/atom_particle/electron.html
J.J. Tomson was the man that in 1897 discovered that an atom has negatively charged parts. He thought that these negative parts are in the atom just kind of floating around but he was wrong. He also relized that an atom has a neutral charge which means there must be positively charged parts also. But, back to electrons. They have a very small mass compared to the other parts of an atom. It has a mass of 0.000000000000000000000000000911 grams. Is about 1/836 atomic mass units.They are very small and very light and they are nothing compared to the nucleus of an atom. Electrons can be at different energy levels throughout the atom. They can take in or let go of something called a photon. A photon is particles of light. To learn more about these photons go to http://www.windows2universe.org/physical_science/magnetism/photon.html In the atom these electrons don'tgo in little tracks like some people thought. Erwin Shroedinger found out that these electrons just zoom around the atom where ever they want. There isn't a certain circle they follow the whole time. The Modern Atomic Model has a nucleus in the center and then there is a big area where we think the electrons might be. As you can see these electrons are very interesting but here is some information about the proton.
Protons:
Protons are very close to the size of a neutron and a lot bigger than electrons. It has a mass of 1 atomic mass units.It was discovored in 1897 by J.J. Thomson. It has 1,836 times more mass than an electron. They are positively charged and there are the same number as protons as electrons. You can find how many protons by looking at the atomic number in the upper corner of the atom in the periotic table. A proton is made up of these things called quarks. There are three quarks in every proton. They are up, up, and down. There are six types of quarks and they are up, down, top, bottom, charm, and strange. These quarks are held together by gluons. Protons are in the middle of the atom with the neutron. The neutrons and protons make up the nucleus that has all the mass of an atom.
Here is a drawing of an atom. But remeber that the electrons don't go on set circles they go where they want to go and just sort of bonce around.
This shows a proton and the quarks that are inside of it and that are held together by gluons.
Citations:
I got all my information from http://en.wikipedia.org/wiki/Electrons http://en.wikipedia.org/wiki/Proton http://www.windows2universe.org/physical_science/physics/atom_particle/proton.html http://www.windows2universe.org/physical_science/physics/atom_particle/electron.html
Thursday, September 2, 2010
Citations
Oh, sorry I forgot to add this. I got all my information from wikipedia and from Mr. Harrelson's slide show!
Tuesday, August 31, 2010
Democritus and Rutherford
Democritus:
Democritus was a man around 430 BCE. He was a ancient Greek philosipher and he lived in Greece. He worked on one question and that question was "What is stuff made of?" He assumed it was this thing called matter that was made of small pieces. These pieces were so small that they can't be broken or cut into anything smaller. He said that they are unable to get rid of and always will be there. Also hey said that they are constantly moving and that there is an infinite amount of these things that he named atomos, which means uncuttable, in the universe. Democritus realized that most likely the strongness of an object had something to do with the atom and how it was shapped. He made a drawing of what he assumed was what a atom looked like and it was basically a circle.
Rutherford:
Rutherford was a British-New Zealand scientist. He was a chemist and a physicist that is called the father of nuclear physics. He was a student of Thomson, another famous chemist and physicst, that discoverd that an atom has negatively charged things in an atom so assumed there must be a positively charged thing since atoms are neutral. Rutherford did this project called "Gold Foil" and you can read about this at http://en.wikipedia.org/wiki/Geiger%E2%80%93Marsden_experiment. At the end of this project of his he figured out that most of the mass is in the nucleus and that his teacher was wrong when he drew his model. He named the positivaly charged particles the proton. Rutherford also drew what he thought the atom looked like and that was something that looked like a "Peach Pit." It was a circle with a small circle in the middle (nucleus) and all these little electons on the outside.
Rutherford and Democritus:
Democritus and Rutherford are very different in the way they did things when trying to figure out things. Democritus was in 430 BCE and Rutherford was somewhere around 1911. So, there is a big difference between those years because equitment gets better and people begin to know more and more as the years go on. Yes, they both were chemist and physicist but they still did things differently. For one thing Democritus mostly assumed things after he looked up as much as he could find out Which wasn't much. He did this without doing many experiments while on the other hand Rutherford did experiments like the one I mentioned earlier named "Gold Foil." Also people came between these two men like Dalton and J.J. Thomson, who were very good at this stuff also, so what they found out was of use to Rutherford but not Democritus. This could have given Rutherford an extra advantage over Democritus but they were both really good at this kind of stuff. As you can clearly see these two men are alike and different in what they did and how they found out a lot of the things they did.
Rutherford
Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated positive charge. Note that the image is not to scale, in reality the nucleus is vastly smaller than the electron shell.
"Gold Foil" picture and explanation is from http://en.wikipedia.org/wiki/Geiger%E2%80%93Marsden_experiment
Democritus:
Democritus was a man around 430 BCE. He was a ancient Greek philosipher and he lived in Greece. He worked on one question and that question was "What is stuff made of?" He assumed it was this thing called matter that was made of small pieces. These pieces were so small that they can't be broken or cut into anything smaller. He said that they are unable to get rid of and always will be there. Also hey said that they are constantly moving and that there is an infinite amount of these things that he named atomos, which means uncuttable, in the universe. Democritus realized that most likely the strongness of an object had something to do with the atom and how it was shapped. He made a drawing of what he assumed was what a atom looked like and it was basically a circle.
Rutherford:
Rutherford was a British-New Zealand scientist. He was a chemist and a physicist that is called the father of nuclear physics. He was a student of Thomson, another famous chemist and physicst, that discoverd that an atom has negatively charged things in an atom so assumed there must be a positively charged thing since atoms are neutral. Rutherford did this project called "Gold Foil" and you can read about this at http://en.wikipedia.org/wiki/Geiger%E2%80%93Marsden_experiment. At the end of this project of his he figured out that most of the mass is in the nucleus and that his teacher was wrong when he drew his model. He named the positivaly charged particles the proton. Rutherford also drew what he thought the atom looked like and that was something that looked like a "Peach Pit." It was a circle with a small circle in the middle (nucleus) and all these little electons on the outside.
Rutherford and Democritus:
Democritus and Rutherford are very different in the way they did things when trying to figure out things. Democritus was in 430 BCE and Rutherford was somewhere around 1911. So, there is a big difference between those years because equitment gets better and people begin to know more and more as the years go on. Yes, they both were chemist and physicist but they still did things differently. For one thing Democritus mostly assumed things after he looked up as much as he could find out Which wasn't much. He did this without doing many experiments while on the other hand Rutherford did experiments like the one I mentioned earlier named "Gold Foil." Also people came between these two men like Dalton and J.J. Thomson, who were very good at this stuff also, so what they found out was of use to Rutherford but not Democritus. This could have given Rutherford an extra advantage over Democritus but they were both really good at this kind of stuff. As you can clearly see these two men are alike and different in what they did and how they found out a lot of the things they did.
Rutherford
"Gold Foil"
Top: Expected results: alpha particles passing through the plum pudding model of the atom undisturbed.
Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated positive charge. Note that the image is not to scale, in reality the nucleus is vastly smaller than the electron shell.
"Gold Foil" picture and explanation is from http://en.wikipedia.org/wiki/Geiger%E2%80%93Marsden_experiment
Democritus:
Wednesday, August 25, 2010
Subscribe to:
Comments (Atom)
FINGER MONKEY